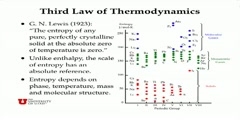
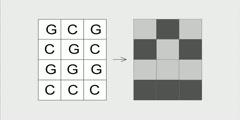
Lec 80 - More on Entropy
More on Entropy More clarification as to what entropy is and what entropy is not.
Video is embedded from external source so embedding is not available.
Video is embedded from external source so download is not available.
Channels: Chemistry (General)
Uploaded by: khanchemistry ( Send Message ) on 10-09-2012.
Duration: 8m 56s
Here is the next lecture for this course
What is Entropy and Heat Engines
06:50 | 6172 viewsWhat is Entropy and Equilibrium
08:21 | 5072 viewsAll About Heat And Entropy
08:40 | 4936 viewsLec Last - The Second Law of Thermodynami ...
01:11:48 | 3386 viewsLec 36 - Bond Energies, the Boltzmann Fac ...
47:51 | 4360 viewsLec 75 - Proof: S (or Entropy) is a valid ...
15:38 | 3370 viewsLec 76 - Thermodynamic Entropy Definition ...
15:38 | 5168 viewsLec 77 - Reconciling Thermodynamic and St ...
28:26 | 3131 viewsLec 78 - Entropy Intuition
19:15 | 3095 viewsVisual DNA entropy1
00:21 | 5379 viewsVisual DNA entropy2
00:20 | 5314 viewsLec 9 - MIT 5.60 Thermodynamics & Kinetic ...
50:07 | 2960 viewsLec 10 - MIT 5.60 Thermodynamics & Kineti ...
52:45 | 2839 viewsLec 6 - Topics in String Theory
01:00:23 | 2636 viewsLec 7 - Topics in String Theory
01:42:17 | 2945 viewsNo content is added to this lecture.
This video is a part of a lecture series from of khan
Lecture list for this course
Lec 2 - Introduction to the atom
Lec 4 - More on orbitals and electron configuration
Lec 5 - Electron Configurations
Lec 6 - Electron Configurations 2
Lec 8 - Groups of the Periodic Table
Lec 9 - Periodic Table Trends: Ionization Energy
Lec 10 - Other Periodic Table Trends
Lec 11 - Ionic, Covalent, and Metallic Bonds
Lec 12 - Molecular and Empirical Formulas
Lec 13 - The Mole and Avogadro's Number
Lec 14 - Formula from Mass Composition
Lec 15 - Another mass composition problem
Lec 16 - Balancing Chemical Equations
Lec 18 - Stoichiometry: Limiting Reagent
Lec 19 - Ideal Gas Equation: PV=nRT
Lec 20 - Ideal Gas Equation Example 1
Lec 21 - Ideal Gas Equation Example 2
Lec 22 - Ideal Gas Equation Example 3
Lec 26 - States of Matter Follow-Up
Lec 27 - Specific Heat, Heat of Fusion and Vaporization
Lec 28 - Chilling Water Problem
Lec 31 - Covalent Networks, Metallic, and Ionic Crystals
Lec 33 - Suspensions, Colloids and Solutions
Lec 35 - Boiling Point Elevation and Freezing Point Supression
Lec 36 - Introduction to Kinetics
Lec 37 - Reactions in Equilibrium
Lec 38 - Mini-Video on Ion Size
Lec 39 - Keq Intuition (mathy and not necessary to progress)
Lec 40 - Keq derivation intuition (can skip; bit mathy)
Lec 41 - Heterogenous Equilibrium
Lec 42 - Le Chatelier's Principle
Lec 43 - Introduction to pH, pOH, and pKw
Lec 44 - Acid Base Introduction
Lec 45 - pH, pOH of Strong Acids and Bases
Lec 48 - Conjugate Acids and Bases
Lec 49 - pKa and pKb Relationship
Lec 50 - Buffers and Hendersen-Hasselbalch
Lec 51 - Strong Acid Titration
Lec 53 - Half Equivalence Point
Lec 55 - Introduction to Oxidation States
Lec 56 - More on Oxidation States
Lec 57 - Hydrogen Peroxide Correction
Lec 62- Exponential Decay Formula Proof (can skip, involves Calculus)
Lec 63 - Introduction to Exponential Decay
Lec 64 - More Exponential Decay Examples
Lec 65 - Macrostates and Microstates
Lec 66 - Quasistatic and Reversible Processes
Lec 67 - First Law of Thermodynamics/ Internal Energy
Lec 68 - More on Internal Energy
Lec 70 - PV-diagrams and Expansion Work
Lec 71 - Proof: U=(3/2)PV or U=(3/2)nRT
Lec 72 - Work Done by Isothermic Process
Lec 73 - Carnot Cycle and Carnot Engine
Lec 74 - Proof: Volume Ratios in a Carnot Cycle
Lec 75 - Proof: S (or Entropy) is a valid state variable
Lec 76 - Thermodynamic Entropy Definition Clarification
Lec 77 - Reconciling Thermodynamic and State Definitions of Entropy
Lec 81 - Efficiency of a Carnot Engine
Lec 82 - Carnot Efficiency 2: Reversing the Cycle
Lec 83 - Carnot Efficiency 3: Proving that it is the most efficient
Lec 86 - Hess's Law and Reaction Enthalpy Change
Lec 87 - Gibbs Free Energy and Spontaneity
Lec 88 - Gibbs Free Energy Example
Lec 89 - More rigorous Gibbs Free Energy/ Spontaneity Relationship
Lec 90 - A look at a seductive but wrong Gibbs/Spontaneity Proof
Lec 91 - Stoichiometry Example Problem 1
Lec 92 - Stoichiometry Example Problem 2
Lec 93 - Limiting Reactant Example Problem 1
Lec 94 - Empirical and Molecular Formulas from Stoichiometry
Lec 95 - Example of Finding Reactant Empirical Formula
Lec 96 - Stoichiometry of a Reaction in Solution
Lec 97 - Another Stoichiometry Example in a Solution
Lec 98 - Molecular and Empirical Forumlas from Percent Composition