Lec 86 - Hess's Law and Reaction Enthalpy Change
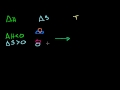
Hess's Law and Reaction Enthalpy Change Using Hess's Law and standard heats of formation to determine the enthalpy change for reactions
Video is embedded from external source so embedding is not available.
Video is embedded from external source so download is not available.
Channels: Chemistry (General)
Tags: Hess's Law and Reaction Enthalpy Change
Uploaded by: khanchemistry ( Send Message ) on 10-09-2012.
Duration: 15m 40s
Here is the next lecture for this course
Lec 115 - Standard Enthalpy of Change
02:04 | 1862 viewsExothermic Reaction Molten Magic
00:36 | 8897 viewsChemical Reactions- Changes in Enthalpy
08:20 | 5840 viewsAn Activity to Show Factors Affecting Rat ...
02:29 | 4824 viewsLec 104 - Change of State Example
07:38 | 2131 viewsChemical Science - Bond Energies / Bond E ...
47:24 | 24922 viewsPhysical reaction to stress and polygraph
02:57 | 9289 viewsReaction of Sodium & Chlorine (with subti ...
00:51 | 6484 viewsPhotosynthetic Reaction Center
01:07 | 8704 viewsCondensation Reaction
08:59 | 9542 viewsDiels-Alder Reaction- Highest Occupied Or ...
00:16 | 7549 viewsDemonstration on Endothermic Reaction
03:06 | 10869 viewsExothermic Reaction
00:53 | 8623 viewsExothermic Reaction With Sodium Acetate
02:05 | 15096 viewsIdentifying pH change
04:11 | 6213 viewsNo content is added to this lecture.
This video is a part of a lecture series from of khan