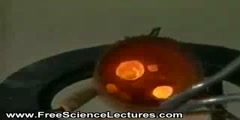
Exothermic Reaction With Sodium Acetate
For laboratory usage, sodium acetate is inexpensive, and it is usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid with sodium carbonate, sodium bicarbonate, or sodium hydroxide. These reactions produce aqueous sodium acetate, and some water. Carbon dioxide is produced in the reaction with sodium carbonate and bicarbonate, and it leaves the reaction vessel as a gas (unless the reaction vessel is pressurized). This is the well-known "volcano" reaction between baking soda and vinegar.
Channels: Chemistry (General)
Tags: Exothermic Reaction With Sodium Acetate
Uploaded by: buraktube ( Send Message ) on 09-11-2010.
Duration: 2m 5s