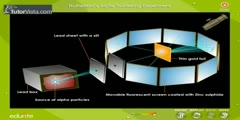
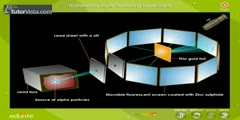
Rutherford's Experiment: Nuclear Atom
A beam of alpha particles, generated by the radioactive decay of radium, was directed normally onto a sheet of very thin gold foil. The gold foil was surrounded by a circular sheet of zinc sulfide (ZnS) which was used as a detector: the ZnS sheet would light up when hit with alpha particles. Under the prevailing plum pudding model, the alpha particles should all have been deflected by, at most, a few degrees; measuring the pattern of scattered particles was expected to provide information about the distribution of charge within the atom. However they observed that a very small percentage of particles were deflected through angles much larger than 90 degrees. According to Rutherford: ''It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in
Channels: Experiments Chemistry (General) Analytical chemistry
Tags: chemistry science physics rutherford ernest nuclear atom experiment nucleus gold foil
Uploaded by: jamvj ( Send Message ) on 07-06-2009.
Duration: 0m 47s