Rutherford
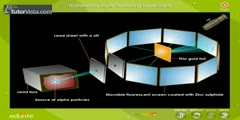
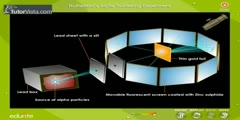
In 1911, Rutherford came forth with his own physical model for subatomic structure, as an interpretation for the unexpected experimental results. In it, the atom is made up of a central charge (this is the modern atomic nucleus, though Rutherford did not use the term "nucleus" in his paper) surrounded by a cloud of orbiting electrons. In this 1911 paper, Rutherford only commits himself to a small central region of very high positive or negative charge in the atom, but uses the following language for pictorial purposes: "For concreteness, consider the passage of a high speed α particle through an atom having a positive central charge N e, and surrounded by a compensating charge of N electrons. [1] From purely energetic considerations of how far α (alpha) particles of known speed would be able to penetrate toward a central charge of 100 e, Rutherford was able to calculate that the radius of his gold central charge would need to be less (how much less could not be told) than 3.4 x 10-14 metres (the modern value is only about a fifth of this). This was in a gold atom known to be 10-10 metres or so in radius--- a very surprising finding, as it implied a strong central charge less than 1/3000th of the diameter of the atom. The Rutherford model didn't attribute any structure to the orbits of the electrons themselves, though it did mention the atomic model of Hantaro Nagaoka, in which the electrons are arranged in one or more rings. The Rutherford paper suggested that the central charge of an atom might be "proportional" to its atomic mass in hydrogen mass units (roughly 1/2 of it, in Rutherford's model). For gold, this mass number is 197 (not then known to great accuracy) and was therefore modeled by Rutherford to be possibly 196. However, Rutherford did not attempt to make the direct connection of central charge to atomic number, since gold's place on the periodic table was known to be about 79, and Rutherford's more tentative model for the structure of the gold nucleus was 49 helium nuclei, which would have given it a mass of 196 and charge of 98. This differed enough from gold's "atomic number" (at that time merely its place number in the periodic table) that Rutherford did not formally suggest the two numbers might be exactly the same.
Channels: Chemistry (General) Physics (General) Atomic, molecular, and optical physics
Tags: Rutherford
Uploaded by: jamvj ( Send Message ) on 07-06-2009.
Duration: 1m 24s