Exothermic Reaction Molten Magic
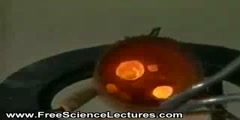
There are other chemical reactions which must absorb energy in order to proceed. These are endothermic reactions. Endothermic reactions cannot occur spontaneously. Work must be done in order to get these reactions to occur. When endothermic reactions absorb energy, a temperature drop is measured during the reaction. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+ΔH) Many chemical reactions release energy in the form of heat, light, or sound. These are exothermic reactions. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (ΔS > 0) of the system. They are denoted by a negative heat flow (heat is lost to the surroundings) and decrease in enthalpy (ΔH < 0). In the lab, exothermic reactions produce heat or may even be explosive.
Channels: Chemistry (General)
Tags: Exothermic Reaction Molten Magic
Uploaded by: buraktube ( Send Message ) on 09-11-2010.
Duration: 0m 36s