Galvanic Cell Animation (Zn/Cu)
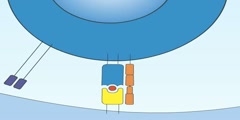
The Galvanic cell, named after Luigi Galvani, is a part of a battery consisting of an electrochemical cell with two different metals connected by a salt bridge or a porous disk between the individual half-cells. It is sometimes also called a Voltaic cell. Common usage of the word battery has evolved to include a single Galvanic cell, but the first batteries had many Galvanic cells. The Galvanic cell should not be confused with the electrolytic cell, which decomposes chemical compounds by means of electrical energy. A Galvanic cell consists of two half-cells. Each half-cell consists of the following: An electrode, which in the figure are the plates of zinc (Zn) and copper (Cu). An electrolyte, which in the figure are aqueous solutions of zinc sulfate (ZnSO4) and copper(II) sulfate (CuSO4). For the Daniell cell, depicted in the figure, the zinc atoms have a greater tendency to go into solution than do the copper atoms. More precisely, the electrons on the zinc electrode have a higher energy than the electrons on the copper electrode. Because the electrons have a negative charge, to pass them on the zinc electrode must have a more negative electrical potential than the copper electrode. However, in the absence of an external connection between the electrodes, no current can flow. When the electrodes are connected externally — as in the figure, with wire and a voltmeter, the electrons tend to flow from the more negative electrode (zinc) to the more positive electrode (copper). Because the electrons have negative charge, this produces an electric current that is opposite to the electron flow. At the same time, an equal ionic current flows through the electrolyte. For every two electrons that flow from the zinc electrode through the external connection to the copper electrode, on the electrolyte side a zinc atom must go into solution as a Zn2+ ion, at the same time replacing the two electrons that have left the zinc electrode by the external connection. By definition, the anode is the electrode where oxidation (removal of electrons) takes place, so in this galvanic cell the zinc electrode is the anode. Because the copper has gained two electrons from the external connection, it must release two electrons at the electrolyte side, where a Cu2+ ion, from the copper(II) sulfate, plates onto the copper electrode. By definition, the cathode is the electrode where reduction (gain of electrons) takes place, so the copper electrode is the cathode. Electrons will flow from the anode to the cathode. An essential piece of a Galvanic Cell is the salt bridge. The salt bridge serves the vital role of allowing each half-cell's charge to be balanced while preventing the solutions from mixing with each other. Should the solutions be mixed, the reaction will be purely chemical and will not require electrons to flow through the wire — thus, no electricity can be harbored from the cell. Should the charges not be balanced, the anode will have an abundance of positive ions, and the cathode will have an abundance of negative ions. Electrons will not flow with this imbalance. The salt in the salt bridge, however, will ionize and neutralize the charges in each half-cell, allowing the reaction to proceed.
Channels: Scientific Animations Chemistry (General)
Tags: Science HSC Chemistry Model
Uploaded by: jamvj ( Send Message ) on 03-07-2009.
Duration: 0m 24s