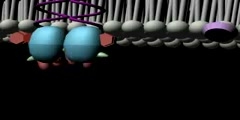
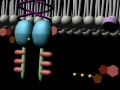
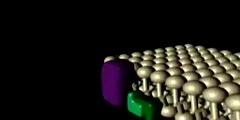
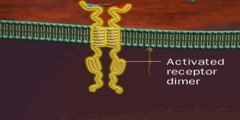
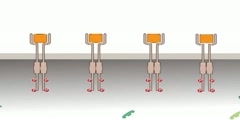
TrK Receptor Animation
Trk receptors are a family of tyrosine kinases that regulates synaptic strength and plasticity in the mammalian nervous system. Trk receptors affect neuronal survival and differentiation through several signal cascades. However, the activation of these receptors also has significant effects on functional properties of neurons. The common ligands of trk receptors are neurotrophins, a family of growth factors critical to the functioning of the nervous system. The binding of these molecules is highly specific. Each type of neurotrophin has different binding affinity toward its corresponding trk receptor. The activation of Trk receptors by neurotrophin binding may lead to activation of signal cascades resulting in promoting survival and other functional regulation of cells. The abbreviation trk (often pronounced 'track') stands for tropomyosin-receptor-kinase (and not tyrosine kinase nor tropomyosin-related kinase, as has been commonly mistaken). The family of Trk receptors is named for the oncogene trk, whose identifical led to the discovery of its first member, TrkA. trk, initially identified in a colon carcinoma, is frequently (25%) activated in thyroid papillary carcinomas . The oncogene was generated by a mutation in chromosome 1 that resulted in the fusion of the first seven exons of tropomyosin to the transmembrane and cytoplasmic domains of the then-unknown TrkA receptor . Normal Trk receptors do not contain amino acid or DNA sequences related to tropomyosin. The three most common types of trk receptors are trkA, trkB, and trkC. Each of these receptors types has different binding affinity to certain types of neurotrophins. The differences in the signaling initiated by these distinct types of receptors are important for generating diverse of biological responses. Neurotrophin ligands of Trk receptors are processed ligands, meaning that they are synthesized in immature forms and then transformed by protease cleavage. Immature neurotrophins are specific only to one common p75NTR receptor. However, protease cleavage generates neurotrophins that have higher affinity to their corresponding Trk receptors. These processed neurotrophins can still bind to p75NTR, but at a much lower affinity.