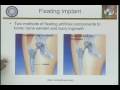
Lec 2 - ME C117 Biocompatibility/FDA Regulatory Agency
ME C117 Biocompatibility/FDA Regulatory Agency Bio Engineering/ME C117: Structural Aspects of Biomaterials - Professor Lisa Pruitt This course provides an overview of medical devices, FDA regulatory issues, biocompatibility and sterilization technology. It examines biomechanical properties: isotropy/anisotropy, stiffness, bending stresses, contact stresses, multiaxial loading, plasticity, fatigue, fracture, wear, corrosion, design issues. Also covered: Orthopedics, Dental, Cardiovascular, and Soft Tissue Reconstruction. Professor Pruitt's current research is focused on fatigue and fracture micromechanisms, cyclic damage zones, and evolution of structure due to cyclic loading and environment in advanced polymers and biomaterials; tribology of...
Video is embedded from external source so embedding is not available.
Video is embedded from external source so download is not available.
Channels: Bioengineering
Tags: Education ME C117 webcast uc berkeley cal mechanical engineering course class yt:quality=high
Uploaded by: berkeleybiomate ( Send Message ) on 04-09-2012.
Duration: 81m 10s
Here is the next lecture for this course
Lec - 19 ME C117 In Class Demonstrations ...
01:22:50 | 2634 viewsLec 1 - ME C117 Course Overview
01:22:10 | 2735 viewsLec - 3 ME C117 Orthopedics
01:20:25 | 2511 viewsLec - 4ME C117 Case Study: Sulzer Recall
01:19:44 | 3392 viewsLec - 5ME C117 Case Study: Hip Implant C ...
01:22:56 | 3087 viewsLec - 6 ME C117 Final Project Outline
01:19:39 | 2870 viewsLec - 7 ME C117 Case Study: Sterilization
01:22:25 | 2956 viewsLec - 8 ME C117 Wear in Total Joint Repl ...
01:20:12 | 2451 viewsLec - 9 ME C117 Total Shoulder Replacemen ...
01:21:16 | 2376 viewsLec - 10 ME C117 Contact Stress in Devic ...
01:19:44 | 3005 viewsLec - 11 ME C117 Dr. Michael Ries, Chief ...
01:04:02 | 2354 viewsLec - 12 ME C117 UHMWPE Fatigue
01:16:21 | 2511 viewsLec - 13 ME C117 Fatigue Design
01:16:43 | 2693 viewsLec - 14 ME C117 Guest Lecture - Dr. And ...
01:18:31 | 2431 viewsLec - 15 ME C117 Defect Tolerant Philosophy
01:21:18 | 2411 viewsNo content is added to this lecture.
This video is a part of a lecture series from of berkeley
Lecture list for this course
Lec 1 - ME C117 Course Overview
Lec - 4ME C117 Case Study: Sulzer Recall
Lec - 5ME C117 Case Study: Hip Implant Corrosion
Lec - 6 ME C117 Final Project Outline
Lec - 7 ME C117 Case Study: Sterilization
Lec - 8 ME C117 Wear in Total Joint Replacements
Lec - 9 ME C117 Total Shoulder Replacements; Contact...
Lec - 10 ME C117 Contact Stress in Devices; Stress...
Lec - 11 ME C117 Dr. Michael Ries, Chief of...
Lec - 12 ME C117 UHMWPE Fatigue
Lec - 13 ME C117 Fatigue Design
Lec - 14 ME C117 Guest Lecture - Dr. Andy Kohm, Spinal...
Lec - 15 ME C117 Defect Tolerant Philosophy
Lec - 16 ME C117 Prof. Rob Ritchie, Fracture in...
Lec - 17 ME C117 Dental Materials
Lec - 18 ME C117 Guest Lecture: Vascular Mechanics
Lec - 19 ME C117 In Class Demonstrations: Knee, Hip,...
Lec - 20 ME C117 Dr. Scott Robertson, LBL - Stents:...
Lec - 21 ME C117 Dr. Alan Pelton, Nitinol Device...
Lec - 22 ME C117 Soft Tissue Reconstruction
Lec - 23 ME C117 Exam Solutions