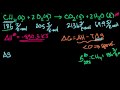Chemical Science - Free Energy of Formation - Lecture 18
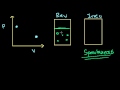
Principles of Chemical Science/n * Email this page/nVideo Lectures - Lecture 18/nTopics covered: /nFree Energy of Formation ΔGof/nInstructor: /nProf. Sylvia Ceyer/nTranscript - Lecture 18/nWe also saw, or we tried to understand what the free energy actually was./nAnd we said that when you have, for example, an exothermic reaction that not all of that energy released is going to be released in a form that can do useful work./nSome of that energy that is released, some of that delta H, for example, goes into, or can go into the vibrations and rotations of the product molecules that energy of which is not able to do useful work./nAnd so the amount of energy that we can actually use to do work is this free energy, is delta G right here./nSo it can be something less than delta H. We also saw a condition, which was the oxidation of glucose, where delta G was actually greater than delta H. And that is the case where we have some of that internal energy locked up in the reactants which now is released in the form of this useful work delta G./nAnd I think we had just started to talk about this term delta S, which is the change in the entropy for the reaction./nAnd we talked about how to calculate it. We calculate it from the absolute entropies of the reactants in the products. Delta S for a reaction then is the absolute entropy of each one of a product./nTimes the appropriate stoichiometric number summed over all the products./nAnd then minus the absolute entropy of each one of the reactants times that appropriate stoichiometric number summed over all the reactants. And it is the difference between the two, delta S for the chemical reaction./nAnd S is an absolute entropy that is a result of the third law of thermodynamics, which I mentioned, I think, last time./nYou will talk about it a lot in detail in 5.60. Let's look at an example here at the change in entropy./nBecause I want to show you that you can have a change in entropy that is large enough to make an endothermic reaction spontaneous. You can have what we call an entropy-driven reaction. For example, the melting of ice./nTo melt ice, to go from the solid form of water to the liquid form, that is an endothermic reaction, 6.95 kilojoules per mole./nHowever, going from the solid form of water to the liquid form there is a great increase there in the entropy, in the disorder. And it is that increase in the disorder that allows that reaction to be spontaneous./nWe can calculate the entropy change for that reaction./nThat entropy change then is the absolute entry of the product, which is liquid water, minus the absolute entropy of the reactant, which is solid water. And you can see that delta S here is positive./nIs there a question I can help you out with? Did you have a question? Can I help you out?/nOK. That delta S is positive. We have increased the disorder of the system. And it is that positive delta S then that gives us a delta G that is negative, meaning our reaction in this direction from solid to the liquid is a spontaneous reaction./nDelta G here is delta H, 6.95 minus the temperature at which the reaction is proceeding times that change in the entropy./nAnd, in this case here, delta G now is minus 1.57 kilojoules. That reaction is spontaneous, even though it is endothermic./nAnd it is that increase in the entropy that is driving this reaction here. Now, I want you also to notice something right here. And that is that the change in the entropy usually is in units of joules per degree Kelvin mole./nDelta H, just about always, is in units of kilojoules per mole./nRemember when you're working with delta H, bells ought to go off in your head. Remember that you probably have to do a unit conversation here from joules to kilojoules. This catches a lot of students on an exam, so I am trying to give you some fair warning about this./nNow, what I want to talk about is this free energy of formation, delta G sub f knot./nThe Gibbs free energy of formation is analogous to the enthalpy of formation. This is what we looked at the last time./nThe Gibbs free energy of formation, delta G sub f knot for R standard state, which is one pressure, is the free energy formation of one mole of a compound from its elements in their most stable form at the standard state./nIt is analogous to the heat of formation, only it's the free energy of formation./nIt is tabulated for you just like the enthalpy of formation is. You can look up the free energy of formation for every compound that is known./nHowever, unlike the enthalpy of formation here, the free energy of formation you can also calculate. You can calculate from this expression, delta G equal delta H minus T delta S./nIf you've got a reaction for which you have defined the free energy of formation, so if you know the free energy of formation of a molecule and you know the delta S for the reaction that defines that enthalpy of formation then you can calculate the free energy of formation for every molecule, which is different from the enthalpy of formation./nThat you do have to look up, but you can actually calculate the free energy of formation given that you know the enthalpy of formation and given that you know delta S for the reaction that defines that enthalpy of formation./nQuestions on that? OK. But why is this free energy of formation so important? Well, that's what we have got to look at right now./nHere is a reaction that produces one mole of carbon dioxide. The delta G for this reaction is minus 394 kilojoules per mole./nThe delta G for this reaction is defined as the free energy of formation for CO2. Why? Because this reaction, as written, produces one mole of CO2 from the elements that compose CO2./nBut the elements in their most stable form./nThe elements are carbon. And the most stable form of carbon is graphite. And the element is oxygen. NO2 is the most stable form of oxygen at one bar pressure and room temperature which we are working with here./nThat is the free energy of formation of CO2./nAnd this is very important because that free energy of formation of a molecule defines the molecule's stability relative to decomposition to its elements./nFor example, the forward direction here, this delta G is negative. It is spontaneous in the forward direction. When delta G, a formation, is negative for a molecule, what we say is that the molecule is thermodynamically stable relative to its elements./nBecause the formation of that molecule, that delta G is negative./nThe reaction as spontaneous is written in the forward direction. It is not spontaneous in the reverse direction. The reverse direction, this delta G would be positive./nSo because the delta G for this reaction is negative, we say that CO2 is thermodynamically stable relative to decomposition to its elements, carbon and oxygen./nThis is important. The sign of the free energy of formation for a molecule is important. Had the free energy of formation of CO2 been positive, we would have said that was thermodynamically unstable relative to decomposition to its elements./nBecause this forward reaction, delta G would have been positive, but the reverse reaction would have been negative./nReverse reaction, there would be a spontaneous tendency for CO2 to decompose. But this is an example where CO2 is stable relative to decomposition to its elements./nHere is an example of a molecule where it is thermodynamically unstable relative to decomposition to its elements, benzene./nI write a reaction here that is the formation of one mole of benzene from the elements in the most stable form. The free energy of formation of a mole of benzene here is positive, 124 kilojoules per mole./nIt is positive./nWhat does that mean? Well, it means that the reverse reaction delta G is negative. It means that benzene has a thermodynamic tendency to decompose into its elements. It is unstable relative to decomposition into its elements because it has a positive delta G./nIt is the reverse reaction that is spontaneous./nBut even though a reverse reaction may be spontaneous, it can also be really pretty slow. When was the last time you saw a pint of benzene decompose to graphite and hydrogen? Not recently./nAnd so even though you have a thermodynamic tendency to decompose, it doesn't mean that the rate is going to be fast enough for you to see that in any reasonable amount of time./nThere is a thermodynamic effect and there is a kinetic effect, and you are going to talk about the kinetic effect in much more detail with Professor Drennan a few weeks from now./nBut, at the moment right here, we've got two different names to talk about, thermodynamic stability and kinetic stability, so to speak. And that is the following./nWe call a molecule stable or unstable./nAnd when we use those terms, stable and unstable, we are referring to the delta G of formation. We are referring to the thermodynamic tendency of the molecule to decompose. So benzene here is thermodynamically unstable with respect to decomposition to its elements./nHowever, benzene is what we call nonlabile./nThese terms, labile and nonlabile, refer to the rate with which that thermodynamic tendency is realized. Because the rate of decomposition is so slow such that you never see it, we say benzene is nonlabile./nNonlabile means it is not going to decompose./nNot because it doesn't have the thermodynamic tendency to, but because the rate is just too slow. However, if benzene's rate for decomposition was very fast we would call it a labile molecule./nBut it is not. It is nonlabile, but it is unstable. Unstable refers to thermodynamic tendency. Labile and nonlabile refers to the kinetic, the rate at which that thermodynamic tendency is realized./nWell, like the entropy of formation, delta H sub f, delta G sub f, the free energy of formation, well, that can also be zero./nFor example, for the elements hydrogen, oxygen, chlorine, xenon, all those gases, free energy of formation is zero./nJust the same way in which we described it last time for the enthalpy of formation./nFor the elements bromine here and mercury in the liquid form, delta G sub f, free energy of formation is equal to zero. For the elements carbon in the form of graphite, sodium iron, iodine, all solids, their free energy of formation is also equal to zero./nBecause those are the elements in their most stable form at R standard state./nHowever, look at this. The free energy of formation of bromine in the gas phase, that is not equal to zero. It is not equal to zero because bromine in the gas phase is not the most stable form of bromine at R standard state./nLiquid brome is./nDelta G, a formation of bromine in the gas phase, is not zero. Likewise, here the free energy of formation of diamond, that is not zero, because diamond is not the most stable form of the element carbon at one bar pressure at room temperature./nGraphite is. It is important to look at the phase of the elements that you are dealing with./nDo you want to calculate delta G for a reaction? Well, just like calculating delta H for the reaction, you can use the free energy of formation./nYou need to know the free energy of formation for every one of the products, multiply that by the appropriate stoichiometric number and sum that all up./nAnd then you do the same here for the reactants./nYou need to know the free energy of formation for every single one of the reactants, multiplied by the stoichiometric number, sum that all up and take the difference, and you will have the free energy for that chemical reaction./nNotice this is products minus reactants./nJust like calculating enthalpies from the heats of formation, that was products minus reactants. However, when we calculated enthalpies from bond enthalpies that was reactants minus products./nThat is something you do have to know. Likewise, you can also calculate delta G for a reaction now from knowing delta H for that reaction and also knowing delta S for that reaction./nYou've got your choice, to use the free energies of formation that you can look up or from knowing the enthalpy for a reaction and knowing the entropy for that reaction you can calculate delta G./nYou've got your choice with delta G here, depending on what information you're given or know./nNow what we want to talk about is our ability or inability to control the spontaneity of some chemical reaction to control it by virtue of adjusting the temperature./nLet's take this example here. This is sodium bicarbonate, better known as baking soda./nThis is what you put into the dough of some kind of baked goods that you want to make, muffins or cakes. You put it in there in order to lighten the batter./nAnd, of course, the way this works is that the sodium bicarbonate decomposes. It decomposes to form CO2 and water./nAnd when it decomposes it does so in the oven and the CO2 and the water then expand, they evaporate, but in that dough they kind of make bubbles before they totally evaporate./nAnd around those gas bubbles the dough kind of hardens a little bit and eventually the CO2 and water are driven off./nBut what it leaves behind is a very porous structure in the dough. It leaves you something that you can actually put your teeth into./nHave you ever tried putting your teeth into some cake where somebody left out the sodium bicarbonate? It is an interesting experience. I have done it. Important here. But this is a reaction that is very endothermic, plus 136 kilojoules per mole./nIt is, however, a reaction where there is an increase in the entropy a lot here, increasing the disorder. And let's calculate delta G for this reaction. Well, delta G for this reaction at room temperature is plus 36 kilojoules per mole./nIt is non-spontaneous./nYou know what? That is good because you don't want that reaction to start when the dough batter is still sitting on the kitchen counter because at that point you are not going to be able to harden the dough in any way./nBut let's try to make this reaction now spontaneous by increasing the temperature. Let's make this term here negative, more in absolute value./nWe want to make this term larger than delta H so that we can have a negative delta G./nWell, we can do that by raising the temperature to a baking temperature 350 Fahrenheit, which is about 450 degrees Kelvin. Now, when we calculate delta G at 450 degree Kelvin, what we find is that delta G is now negative./nAnd now we've got a spontaneous reaction./nNow when you get up to this baking temperature this reaction proceeds readily in the forward direction. We have adjusted the spontaneity of this particular reaction by increasing the temperature by making this second term, the T times delta S, larger in absolute magnitude than delta H./nWe have made that reaction spontaneous./nThere are reactions for which we can control the spontaneity by adjusting the temperature. And let's take a look at that. Well, as you know here, delta G is the linear function of the temperature./nLet me just draw a dependence. And, actually, this is for the decomposition of sodium bicarbonate. Let me just draw delta G as a function of the temperature./nThe slope of this line here is minus delta S for that reaction./nYou can see that. This is going to be the slope. I am plotting it versus T. The intercept is delta H. It is the enthalpy change for this reaction. And now let me just draw a zero here, a dotted line./nI drew that zero because I want you to see that at some temperature right here, that I am going to label T star, that delta G is equal to zero./nWhat that means is that at this temperature the sign of delta G is changing. You can see that for temperatures less than T star the value of delta G knot here is greater than zero, meaning the reaction is non-spontaneous./nFor temperatures greater than T star right in here, you see that delta G knot is less than zero./nIt means we have a spontaneous reaction. We have controlled the spontaneity of this reaction by the use of temperature. Let's calculate the value of the temperature at which the spontaneity of this reaction changes./nLet's do that./nTo do that we are going to set delta G knot here equal to zero and then we are just going to solve for T star. We are going to solve for that value of T star that for this delta H and that delta S will make delta G knot equal to zero./nLet's do that. Well, then T star is delta H over delta S./nIf I plug in for the decomposition of sodium bicarbonate, the delta H and the delta S, then T star is 406 degrees Kelvin. So your sodium bicarbonate is not going to decompose until you get to this temperature./nAt least for this reaction it appears we can control the spontaneity of this chemical reaction./nAnd that is the case. In general, if you have a reaction that is endothermic, that is delta H is greater than zero, and if that reaction increases in entropy, delta S is greater than zero, that reaction will be spontaneous for temperatures greater than some temperature T star./nYou can see that by the signs of the reactions here./nIf delta H up here is positive and delta S is positive then you've got to increase this second term, T delta S, the absolute magnitude of it enough so that it is larger than delta H./nAnd you are subtracting the two, well, that is when you are going to get the delta G negative. For large enough temperatures the delta G will be negative./nBut suppose we have a reaction that is exothermic, delta H is less than zero, and a reaction that decreases the entropy, delta S is less than zero./nI plotted that now as a function of the temperature. And you can see that the slope of this line has changed. We now have this positive slope./nWell, in this particular case you are going to have a spontaneous reaction whenever the temperature is less than some temperature T star./nBecause right here that is our T star. And so for temperatures less than that delta G is negative. For temperatures greater than that delta G is positive. Again, you can see that./nIf delta H is less than zero this delta H will be negative./nAnd delta S here is going to be negative. We are going to have a negative times a negative. That is a positive. And so since delta H is negative, T is going to have to be small. This is going to be a positive term./nT is going to have to be small so that an absolute value it is not larger than delta H in order to get a negative delta G./nHowever, suppose we have a reaction that is exothermic, delta H less than zero, and delta S is greater than zero./nWe are increasing the entropy here. Well, this is the best of all worlds. When you have an exothermic reaction that increases the entropy, this reaction is spontaneous at all temperatures. Delta G is going to be negative for all temperatures./nBecause delta H is negative./nAnd then you have minus T delta S. Well, delta S is positive. So you have a negative number plus a negative number, or a negative number minus a positive number, delta G is always negative. Spontaneous at every temperature for those reactions./nYou cannot control the spontaneity of the reaction. And then, finally, you can have a situation where you have an endothermic reaction, delta H greater than zero./nAnd the worst of all case, delta S less than zero./nYou are decreasing the entropy. You are making the system more ordered. In that case, you have a reaction that is never spontaneous. Again, you can see it from the sign. If delta H is positive and delta S here is negative, a negative times a negative is a positive, so we have a positive number plus a positive number, delta G is positive at all temperatures./nSo with temperature there we also cannot adjust the spontaneity./nThis is very important to understand. Now, what we have been talking about so far is delta Gs that have been delta G knots./nWe have been talking about delta Gs, free energies of formation in our standard state of one bar pressure./nWhat that means, for delta G knot, is that the partial pressures of your reactants and your products, they are all one bar. Delta G knot for a reaction is the free energy change for that reaction when there is one atmosphere of each, or one bar of each of the products and the reactants./nLet's take this famous reaction, argon plus boron going to carbon plus deuterium, A plus B, C to D. This delta G knot here is a delta G for a reaction under the conditions of one bar partial pressure for D, one bar partial pressure for C, one bar partial pressure for B, one bar partial pressure for A./nThat is what that means./nBut, you know what, that's not the situation you usually have. Say, for example, you are going to start this reaction. You start the reaction with one bar of A and one bar of B./nBut, of course, you don't have any C or D yet because you haven't run the reaction./nWhat we've got to be able to do is to calculate delta G for conditions which aren't at the standard state, which aren't at one bar D, one bar C, one bar B, one bar A. We've got to be able to do that./nAnd the way you do that is represented here by this expression./nWhere this expression came from is again a subject of great interesting in 5.60. You will see where this expression comes from when you take 5.60, but right now we are going to use it. This is going to allow us to calculate delta G from knowing the standard state delta G knot./nIt is going to allow us to calculate delta G for the reactants and products at any given pressure./nYou see this is delta G equal delta G knot plus RTLN of the following. This has the partial pressure of product C divided by a reference pressure raised to the appropriate stoichiometric number./nAnd that is multiplied by the partial pressure of the product D divided by a reference pressured raised to the appropriate stoichiometric number./nAnd that is all over the partial pressure of A divided by a reference pressure raised to that appropriate stoichiometric number times the partial pressure of the other reactant over some reference pressure raised to the appropriate stoichiometric number./nThat ratio is something that we call a reaction quotient./nWe call it Q. It is the ratio of instantaneous partial pressures. We might be running a reaction, and at any time the reaction may not be complete. At any time we could stop the reaction./nWe can figure out or measure what the partial pressures of reactants and products are./nAnd we can calculate what this Q is knowing what those are from a measurement. That is what the reaction quotient is. Now, our reference pressure is going to be one bar. What I am going to do, and what you can do in this reaction quotient, is that I am going to put PRF, the reference pressure as ones in here./nAnd so I am not going to explicitly write it out./nAnd that is just fine, as long as you put the pressures then here in units of bar. So this is the reaction quotient, the form of it that we are going to use, as long as you put the pressures here in units of bar./nWe've got to remember this because I am now going to use this in a very interesting way./nThis is going to give me delta G at any arbitrary pressures of the reactants and the products. How am I going to use this? I am going to start to talk about the equilibrium constant for the reaction./nI've got my famous A plus B going to C plus D reaction./nAnd you already know that all reactions have some kind of equilibrium. There is a forward reaction and there is a reverse reaction. And we know that, as we said, delta G is less is zero./nIf delta G were less than zero for this reaction as written then it is the forward reaction that is spontaneous./nIf delta G is greater than zero for the reaction as written then it is the reverse reaction that is spontaneous./nAnd now here comes the really important point, and that is if delta G is equal to zero for that reaction as written then we are at chemical equilibrium./nWhen delta G is equal to zero then we are at equilibrium./nNotice this is not delta G knot equal zero. This is delta G equals zero. That is what defines chemical equilibrium, when that delta G that I just showed you is equal to zero./nHere is that expression again that I showed you./nDelta G is delta G knot plus RTLN of this reaction quotient. What I am saying is that we are at equilibrium when this delta G here, this one is equal to zero. Not that one. This one. If that is the case --/nIf you are at equilibrium then I can take this equation here, and what I am going to do is move delta G knot to this side, and the result is delta G knot is equal to minus RTLN of this reaction quotient./nSee where I am going? Delta G was equal to zero. That let me then solve for delta G knot is equal to minus RTLN of this reaction quotient./nNow, under this condition, when delta G is equal to zero, this reaction quotient Q has a special name./nAnd that special name is a thermodynamic equilibrium constant./nWhen we are at equilibrium, this reaction quotient is that equilibrium constant K. This defines the equilibrium constant. What the equilibrium constant tells us is about the relative proportions here of the products to the reactants./nIf the equilibrium is large, we've got a lot of products./nThe numerator has got to be large relative to the reactants. If the equilibrium constant is small, well, then we've got few products relative to the reactants./nThe quotient, Q, that reaction quotient is equal to the equilibrium constant when delta G is equal to zero. Not delta G not./nAnd so that is where this expression comes from. Delta G knot is equal to minus RT times the log of the equilibrium constant./nIf you know delta G knot for a reaction, you can figure out the equilibrium constant. If you know the equilibrium constant by knowing, say, the partial pressures present of the products and the reactants at equilibrium, you can work backwards and get delta G knot./nNow what we are going to do is rearrange these equations here a little bit./nWe are going to rearrange them in a form so that it will be easy to tell whether or not you're at chemical equilibrium./nWe are going to use this reaction quotient, Q, as a measure of whether or not we are at chemical equilibrium./nWe are going to compare Q to K. When Q is equal to K, we are at equilibrium. Remember what Q is. Q is the ratio of the instantaneous partial pressures of the products to the reactants at any time during the reaction./nK is the ration of the equilibrium partial pressures of the products to the reactants./nNow we are going to work on a formalism that is going to allow us to easily compare Q to K so that we can tell whether or not we're at chemical equilibrium. Let's do that. Here is the expression that I wrote earlier that allowed me to calculate delta G at any arbitrary pressures for the products in the reactants./nHere is the expression that I got when delta G was zero when we were at equilibrium./nAnd then delta G is equal to minus RTLNFK. What I am going to do right now is I am going to take this and substitute it in. Let's do that. I am going to substitute this into this more general form./nWhen I do that, I am going to get minus RTLNFK plus RTLNFQ./nThis is just a simple substitution. But now what do I have? I have the difference between two logs. I have a minus LNK here plus an LNQ here. The difference between logs is the log of that quotient of the arguments of those logs./nSo I have RT times LN of Q over K./nThis is the difference in the logs. That is then the log of the quotient of the arguments of those logs. This is going to be great here because look at what we are going to be able to do./nRemember what Q is, the ratio of instantaneous partial pressures./nAnd you know what K is. The ratio of partial pressure is at equilibrium. Bottom line here is the following. If Q is less than K, what that means is we don't have enough products formed compared to what we should have at equilibrium./nThat is, when Q is less than K, we haven't got enough products compared to what we need for chemical equilibrium./nIf Q is less than K, well, then up here in this expression for delta G we have the log of a number smaller than one. The log of number smaller than one is going to be negative./nIf Q is less than K then our delta G is going to be negative./nAnd notice this is delta G, not delta G knot. If our delta G is negative then the reaction is going to be spontaneous in that forward direction. On the other hand, if we've got a situation where Q is greater than K./nThat is where we have more products than what equilibrium says we should have./nWell, in that case, Q over K is going to be a number greater than one. And the log of a number greater than one is always going to be positive. And so our delta G for the reaction as written is going to be greater than zero./nAnd so the forward reaction is not spontaneous, but the reverse reaction is spontaneous./nAnd what is going to happen is that the chemical reaction will proceed in the reverse direction. It will use up all of those extra products that we had for whatever reason to try to attain equilibrium./nHere is another way to look at it./nI am going to plot here Q versus the time of a reaction. And we know when Q is equal to K we are at chemical equilibrium. Suppose we start in a condition some time equal zero where Q is less than K./nIf Q is less than K what that says is that we don't have enough products compared to what we need for chemical equilibrium./nWhen Q is less than K the reaction is going to proceed in the forward direction./nIt is going to make the products so that we can attain equilibrium, so we can make enough products so that we get Q is equal to K. The reaction will proceed in the forward direction./nIf, however, we start in a situation where Q is greater than K that means we've got too many products compared to what our chemical equilibrium says we should have./nAnd so what is going to happen is that the reverse reaction is going to proceed./nIt is going to use up those products and form more reactants so that it can attain the equilibrium value for the partial pressures of the products and the reactants./nQ is going to be our measure of whether or not a reaction is at chemical equilibrium. Very important. Questions?/nR is the gas constant. It is something that will be given to you, you can look up./nOther questions? OK. See you later this week.
Channels: Chemistry (General)
Uploaded by: mitlectures ( Send Message ) on 16-04-2009.
Duration: 47m 47s