Column Chromatography
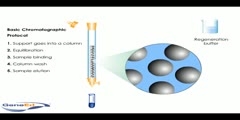
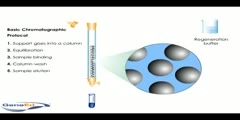
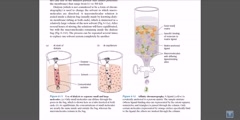
Animation describing the process of column chromatography.Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms. The classical preparative chromatography column is a glass tube with a diameter from 5 to 50 mm and a height of 50 cm to 1 m with a tap at the bottom. A slurry is prepared of the eluent with the stationary phase powder and then carefully poured into the column. Care must be taken to avoid air bubbles. A solution of the organic material is pipetted on top of the stationary phase. This layer is usually topped with a small layer of sand or with cotton or glass wool to protect the shape of the organic layer from the velocity of newly added eluant. Eluant is slowly passed through the column to advance the organic material. Often a spherical eluent reservoir or an eluent-filled and stoppered separating funnel is put on top of the column. Steps involved: During chromatography support or matrix is placed in the column,the support is equilibrated with the buffer which is generally identical to buffer in which the sample is dissolved and sample is applied to the column,in most chromotograhy protocols the protein of interest along with the protein impurities in the sample find to the matrix,the impuritis are washed away by the buffer ,which usually the same buffer which is used in equilibration,then sample is then eluded and collected by washed with appropriate buffer,if teh single buffer is used the process is reffered to as isocratic separation,if multiple buffers are used its is called has gradient,the support is then washed by regeneration buffer that prepares th column for further use and storage with exception of gel filteration ,this basic protocal is used
Channels: Biochemistry
Tags: chromatography matric buffer sample elution isocratic gradient
Uploaded by: sakedi ( Send Message ) on 25-06-2009.
Duration: 1m 20s