Type of radioactive particles
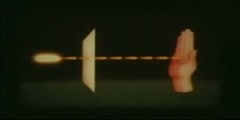
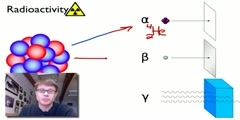
Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting ionizing particles and radiation. This decay, or loss of energy, results in an atom of one type, called the parent nuclide transforming to an atom of a different type, called the daughter nuclide. As for types of radioactive radiation, it was found that an electric or magnetic field could split such emissions into three types of beams. For lack of better terms, the rays were given the alphabetic names alpha, beta and gamma, still in use today. While alpha decay was seen only in heavier elements (atomic number 52 and greater), the other two types of decay were seen in all of the elements. In analyzing the nature of the decay products, it was obvious from the direction of electromagnetic forces that alpha rays carried a positive charge, beta rays carried a negative charge, and gamma rays were neutral. From the magnitude of deflection, it was clear that alpha particles were much more massive than beta particles. Passing alpha particles through a very thin glass window and trapping them in a discharge tube allowed researchers to study the emission spectrum of the resulting gas, and ultimately prove that alpha particles are helium nuclei. Other experiments showed the similarity between beta radiation and cathode rays; they are both streams of electrons, and between gamma radiation and X-rays, which are both high energy electromagnetic radiation. Although alpha, beta, and gamma are most common, other types of decay were eventually discovered. Shortly after discovery of the neutron in 1932, it was discovered by Enrico Fermi that certain rare decay reactions yield neutrons as a decay particle. Isolated proton emission was eventually observed in some elements. Shortly after the discovery of the positron in cosmic ray products, it was realized that the same process that operates in classical beta decay can also produce positrons (positron emission), analogously to negative electrons. Each of the two types of beta decay acts to move a nucleus toward a ratio of neutrons and protons which has the least energy for the combination. Finally, in a phenomenon called cluster decay, specific combinations of neutrons and protons other than alpha particles were spontaneously emitted from atoms on occasion. Still other types of radioactive decay were found which emit previously seen particles, but by different mechanisms. An example is internal conversion, which results in electron and sometimes high energy photon emission, even though it involves neither beta nor gamma decay.
Channels: Chemistry (General) Physics (General) Atomic, molecular, and optical physics
Tags: radioactive particles alpha beta gamma radiation
Uploaded by: jamvj ( Send Message ) on 07-06-2009.
Duration: 2m 49s