Chemical Science - Lewis Diagrams - Lecture 12
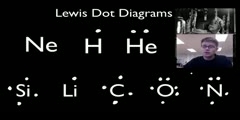
Principles of Chemical Science/n * Email this page/nVideo Lectures - Lecture 12/nTopics covered: /nLewis Diagrams/nInstructor: /nProf. Sylvia Ceyer/nTranscript - Lecture 12/nLast time we were talking about the general interactions that are present in a chemical bond./nThe nuclear-nuclear repulsion. The electron-nuclear attractions. The electron-electron repulsions./nAnd we talked about how the competition of those three interactions are what gave rise to this interaction potential that we drew./nIt is the competition between those interactions that determines what the bond length of a molecule is. It is the competition between those interactions that determines what the well depth is, where the bond strength is in a chemical bond./nWell, today what we're going to do is move on and talk about a specific model for chemical bonding./nAnd the model we're going to talk about is that of Lewis diagrams. Now, interestingly, this is a model that is non-quantum mechanical. This was invented 20 years before quantum mechanics by G. N. Lewis./nSomehow G. N. Lewis recognized, and I don't know how he recognized./nThat's genius, I guess. But he recognized that the key to chemical bonding was a sharing or an overlap of two electrons, one from each atom./nAnd he represented these ideas using these Lewis diagrams in which he took the valence electrons, the total number of valence electrons in the two atoms that were forming a bond./nAnd he redistributed them so that one electron from each atom that was making this bond shared an electron and the rest of the valence electrons were distributed amongst those two atoms such that each atom had an octet of electrons around them./nEach atom had a rare gas electron configuration. And that's the basis of this idea of Lewis diagrams./nThe idea of the Octet Rule. There's no chalk today. I've got a piece. OK, the Octet Rule./nFor example, if you have a chlorine atom with its seven valence electrons here coming together with another chlorine atom with its seven valence electrons to make a fluorine molecule./nWell, the idea is that each one of these extra electrons now on the fluorine, or these unpaired electrons are shared between the two fluorines./nThe result is now, given the sharing, that this fluorine has an octet of electrons around it and this fluorine now has an octet of electrons around it./nThey have this octet configuration which is the lower energy filled shell configuration./nNow, that's the case, of course, for all the elements except for hydrogen. In the case of hydrogen, we're coming in with one atom. Say it's combining or reacting with a chlorine atom. Well, in this case, this electron from the hydrogen is going to be shared with this electron from the chlorine./nAnd now, again, this chlorine has this octet around it./nBut the hydrogen, of course, is just two electrons around it. Its inner gas configuration is that of helium, and so it is satisfied with just two electrons around it. With this sharing idea, Lewis could really understand the Stoichiometry of many compounds./nHe understood why hydrogen gas was H2 and not a hydrogen atom./nHe understood why nitrogen gas was N2 and not a nitrogen atom. He could understand why oxygen was 02 and not an oxygen atom. Now, these Lewis diagrams have absolutely nothing to do with orbitals or wave functions, which we actually know to be a more accurate description of chemical bonding./nWe're going to get to them./nLewis diagrams work 90% of the time to tell you where the electrons are in a molecule. It works so well and it is easy that chemists have used these Lewis diagrams before quantum mechanics and have continued to use them after quantum mechanics./nThey use them because it's easy and it works. It allows you to understand where the electrons are in a chemical bond, on which atoms the electrons are centered, where the lone pairs are, where those electrons that are in the molecule that actually don't participate in the chemical bond./nFor example, here in HCl, these two electrons, these two electrons, these two electrons, they aren't participating in the chemical bond. We call these electrons here lone pairs./nThat's an important definition that we're going to use./nSo we're going to look at this system. Again, it's easy. The alterative is solving the Schrödinger equation, which you don't want to do if you don't have to do because that's hard. You cannot do that quickly./nSo that's the value here. It's going to give us a framework, these Lewis diagrams, to kind of organize a lot of chemical reactions./nTo kind of organize our ideas of bonding and what will react with what./nLet's start doing this. And to that we're going to have a set of rules here. And let me put this front screen down. Actually, on the side screen, could you put my rules up there?/nAll right. The basic idea in Lewis structures is that we're going to take the total number of valence electrons and the two atoms that are going to form this bond./nWe are going to share electrons between the two atoms and then take the rest of the valence electrons and distribute them amongst the two atoms in that molecule such that each atom has an octet configuration./nTo draw a Lewis diagram you're going to have to know what the valence electrons are. In particular, how many valence electrons each atom has./nThis you have to know really off the top of your head./nAnd this is just a quick list of the number of valence electrons. Carbon has four, nitrogen has five, oxygen has six, etc. Let's, as an example, illustrate how to draw these Lewis diagrams. Here are our set of rules./nWe are going to go through them step-by-step./nAs you get better at this, you won't have to go through these rules in such detail. But for now, when we're just learning how to do it, I think this is a very effective way./nSo let's start. Let's take an easy one. We want to draw the Lewis structure of methane. The first thing you have to do is you have to figure out what the skeletal structure is. You have to kind of figure out, as a first pass, what is bonded to what./nAnd now I'm going to give you some hints./nFirst of all, hydrogen, if you have a hydrogen atom. That's always what we call a terminal atom. Terminal means that it is only bonded to one other atom. Chlorine atoms also are usually only terminal atoms./nHydrogen always is. Fluorine is 99% of the time only a terminal atom./nIn the case of methane, which you know has four hydrogens and one carbon, if the hydrogens have to be terminal, well, then there is only one choice here./nYou put the carbon in the center. So that's our skeletal structure. Number two here on our Lewis diagrams, it says count the total number of valence electrons. So let's do that. Hydrogen has one valence electron, but there are four hydrogens./nCarbon has four valence electrons./nThe total number of valence electrons in our molecule here is eight, and I am going to draw those eight valence electrons. Number three here in our rules says count the total number of electrons that you would have to have if each of the atoms had an inner gas configuration./nThese are the number that we've got, eight./nAnd now we are calculating how many we would like to have in the optimal situation if each atom has an inner gas configuration. Let's do that. Well, hydrogen wants two./nAnd we have four hydrogens, so four times two. Carbon wants eight. If each of these atoms had an inner gas configuration we would need 16 electrons./nStep four says to calculate the number of bonding electrons, the way you do that is by taking the total number of electrons that you would have to have if each atom had a noble gas configuration and subtract from it the total number of electrons that you actually do have./nSo we're going to take 16, subtract from that eight. We're going to have eight bonding electrons./nThe way I'm going to represent the eight bonding electrons, or bonding electrons in general is that I am going to draw a red square around my electrons that are bonding electrons./nUp here these are the valence electrons that I've got. It says eight of those valence electrons are bonding electrons, so I am going to draw a box. Those are my bonding electrons. Step five says to assign two bonding electrons to each bond./nWell, let's do that, assign two bonding electrons to each bond./nWell, if we put two there, two there, two there and two there then we've used up all of our valence electrons and all of our bonding electrons in that structure. Number six says if you've got bonding electrons that remain, well, the answer is no, we used them all up./nAnd number seven says do we have any valence electrons left? The answer is no, we don't./nWe've used them all up. So we are done. And you can see the hydrogen has an inner gas configuration around it, the carbon has an inner gas configuration, an octet of electrons around it. Hey, we're done with methane./nBut you could have done that anyway, right? Yes? Yeah. Here is another one./nOh, one other concept here. In this particular structure, carbon is the central atom. What I mean by a central atom is a atom to which everything else is bonded./nSometimes we're going to ask you to write a Lewis structure, and we are going to tell you what atom is the central atom so you know to put it in the center and everything else gets connected to it./nLet's do a higher one./nHere is this molecule. It is going to be hydrogen cyanide, HCn. The first thing you have to do, according to our rule number one, is to draw a skeletal structure. You have a choice here of how to draw that skeletal structure./nYou can put the nitrogen in the center or the carbon in the center./nHydrogen is not going to go in the center because we know the hydrogen is terminal always. What you often do is a first pass when you're drawing the skeletal structure./nYou put the element with the lowest ionization energy in the middle. That is what our rule number one said. Element with lowest ionization energy goes in the middle. That often is true, but not always./nThis is a first pass./nI am going to show you a little bit later some cases where this won't be true and how we are going to determine that the lowest ionization element doesn't go in the center. We're going to have a way to figure this out./nBut for right now, if you had no other choices, that's what you would do, you'd write a structure with the lowest ionization energy element in the center. OK, so we did that./nNumber two, calculate the total number of valence electrons in this molecule./nWell, hydrogen brings one valence electron into the party, carbon brings four and nitrogen brings five. We have a total of ten valence electrons. I am going to draw those ten valence electrons. While you are learning to do this, you might also want to draw ten valence electrons like this./nNext step, calculate the total number of electrons each atom would have to have if, in an ideal world, they had a noble gas configuration without sharing./nWell, the total number of electrons that we would have to have is two for the hydrogen, eight for the carbon and eight for the nitrogen. A total number of 18. Step four says we calculate the number of bonding electrons./nTo do that we subtract the total number that we would have to have for an inner gas configuration, 18, from the total number that we actually do have, which is ten./nThe result is eight bonding electrons. I am going to now draw this red square around the valence electrons that are going to be bonding electrons. Here it goes, we've got eight bonding electrons and two electrons left out here./nWe will see that in a moment, where they go./nNumber five, assign two bonding electrons to each bond. We'll put two there, we put two there, that's great. We've now used up four bonding electrons. We've got four more bonding electrons left. Our next step is do we have any bonding electrons left? We sure do./nWe've got four of them. In that case you start to make double bonds or triple bonds./nA double bond is one in which four electrons are shared. A triple bond is one in which six electrons are shared./nIn general, which atoms do you make double bonds or triple bonds between? Well, in general, double bonds form between carbon, nitrogen, oxygen and sulfur. Triple bonds usually restrict it to carbon, nitrogen and oxygen./nIn this case, we've got a carbon and a nitrogen here, we've got four extra bonding electrons./nWell, what we are going to do is we are going to put these in between the carbon and the nitrogen. We're going to have a triple bond right there. We took care of all our bonding electrons now. Step seven, do we have any valence electrons remaining? We sure do./nWe've got two valence electrons remaining. What do we do with them?/nWell, we assign them as lone pairs. We look to see which atoms don't have an inner gas configuration. Well, hydrogen does. The carbon does now./nIt's got eight electrons around it. But the nitrogen doesn't. It only has six. So we're going to put those two extra valence electrons, as a lone pair, here on the nitrogen. And now the nitrogen has an octet around it./nThese two electrons are called lone pairs./nThey don't participate in the chemical bonding. We did that one. Next one, thionyl chloride. It is SOCl2. How do you do this one? Well, as a first guess, the halogens usually are terminal atoms so they are not bonded to anything else./nThat's usually the case./nNot always, especially for chlorine. We're going to put the chlorines out on the ends somewhere. So then do we put oxygen in the center or sulfur in the center? Well, we're going to pick the lowest ionization energy element and will put that in the center./nThat is going to be sulfur. So we will try this as a skeletal structure./nCount the total number of valence electrons. Chlorine brings seven valence electrons into the party, and there are two chlorine atoms./nSulfur brings six. Oxygen brings six. We've got a total number of 26 valence electrons. Let me draw them all out. Step number three is calculate the total number of electrons that we would have to have for each atom to have an inner gas configuration./nWe've got four atoms here./nEach one of them has got eight. We're going to have 32. That's what we would need for an inner gas configuration for each atom. Let's calculate the total number of bonding electrons. Well, that's 32 minus the number of valence electrons./nThat's six. Of those 26 valence electrons, let me draw my bonding box around six of them./nNext step is to assign those bonding electrons. That's going to be easy. Put two there, two there and two there./nThat means we've just used up our six bonding electrons. Do we have any more left? No, there aren't going to be any double bonds here. However, we've got lots of valence electrons left. We've got 20 of them./nWe're going to have to make lone pairs with those 20 excess valence electrons./nWe're going to have to make ten lone pairs so that each atom has an octet around it. So let's do that. We're going to put six of them around the oxygen, so now the oxygen has an octet, another six, there they go, another six around the chlorine, there they go, another six around this chlorine, there they go./nAnd, finally, we've got two left./nWell, that's going to have to go onto sulfur to make an octet. There it goes. We are done. We've used up all of our valence electrons and we have an octet around each one of the atoms in that molecule./nSo that's the basic procedure for writing these Lewis diagrams. But now I talked about putting the lowest ionization energy element in the center sometimes./nAnd I talked about you've got try this skeletal structure first./nHow do we really know if we've drawn the right Lewis diagram? Well, the way we're going to tell is by this concept called Formal Charge. Formal charge is a measure of the extent to which an atom has gained or lost an electron when it has formed a chemical bond./nSo compared to when it is an atom, the formal charge tells you how much of an electron that has been gained by it or lost by it./nI will make that a little more real in just a moment. How do you assign a formal charge?/nWell, we've got a recipe for this. That formal charge is the following. You count up the number of valence electrons on the free atom./nWe're going to call that V. We're going to subtract from it the number of electrons that are lone pairs on that atom in the molecule, so V minus L. And then the third step is you're going to subtract one-half of the number of bonding electrons that atom has./nOr, one-half the number of electrons that it shares in that molecule./nSo it's V minus L minus S over two. So is the number of electrons it shares. That's the formal charge. Let's illustrate this. Let's take this ion, CN-. I already drew the Lewis structure here, so now what we're going to do is we're going to calculate the formal charge for carbon and the formal charge for nitrogen within this Lewis structure./nFormal charges depend on the Lewis structure./nReady? Let's do it. For carbon, V is four. We have four valence electrons. In this structure L is two. L is the number of lone pair electrons. Look at in this structure./nHere are these electrons, the lone pair that don't participate in the bond./nWe have two lone pair electrons. It's minus two. And then we have to subtract from that minus one-half the number of shared electrons. Well, the number of shared electrons in the case of the carbon is six./nWe've got a triple bond here. That's six over two, that's three. The formal charge on carbon in this Lewis structure is minus one. What we often do is put a minus one somewhere near that carbon and circle it./nThat is the formal charge on the carbon./nWhat that means is that the carbon has gained one more electron than it usually has as an atom. It's a little bit more negatively charged in this molecule than it is in the atom. Let's do nitrogen./nNitrogen, valence electrons five./nThen minus the number of lone pairs. Well, that's two. Here is that lone pair. And then it's minus one-half the number of shared electrons or bonding electrons./nWell, that's three. The result is a formal charge of zero. And so what we would usually do is we'd write a zero with a circle around it near that nitrogen./nSo the formal charge on carbon is minus one, the formal charge on nitrogen is zero in this structure, but now you've got to check to make sure you did everything right./nAnd here is the check. The check is that the total charges here, minus one plus zero is minus one, the sum of the formal charges has to equal the overall charge on the molecule. Minus one plus zero is minus one./nThat does equal the overall charge on the molecule./nSo we did everything right. That is an important thing to check. That's how you calculate formal charge. Now, how do you use it? Well, first of all, before I tell you how you use it, let me just emphasize that formal charge is not oxidation number./nOxidation number does not depend on the Lewis structure./nFormal charge depends on the Lewis structure. You're going to talk about oxidation number with Professor Drennan. If you don't know what it is that is OK./nYou will learn about it in a few weeks. But if you do know what it is, I just want to emphasize that formal charge is not the oxidation number./nHow do we use formal charge to make sure that we've got the correct Lewis structure? That's what you do./n[LAUGHTER] You use the formal charge. What you do is you realize that structures with lower absolute values of formal charge are the more stable structures. Structures which have formal charges on the atoms that are lower are more stable./nStructures where the number of electrons that the atom has gained or lost in forming a molecule is smaller are the stable structures./nThis is really important because you're going to often have a situation where you've got two different skeletal structures. You've obeyed all of the rules for drawing the Lewis diagrams of those two different skeletal structures./nThose two different arrangements of atoms./nBut only one of those structures is going to be correct. And the one that is correct is going to have the lower set of absolute values of the formal charge. So let's illustrate this. Let's take this ion./nIt's the thiocyanate ion, NCS-, as I have written it here./nNow, if you were just told you've got an ion that has nitrogen, carbon and sulfur in it, you might say, well, how do I start? Well, you could start by putting the element with the lowest ionization energy in the center./nThat would be a good place to start. You should do that. But what we're going to do is we're going to look at all three possibilities here./nWe're going to look at a Lewis structure where the carbon is in the middle, where the sulfur is in the middle, and now where the nitrogen is in the middle./nAnd we drew these Lewis structures. I've already drawn them here for you according to our rules posted here on these walls. But only one of these structures is correct. And we're going to use the idea of formal charges to determine which one is correct, which one has the lowest energy./nIf we take this structure and we calculate the formal charge for nitrogen, we will find it is minus one./nThe formal charge on the carbon here is zero. The formal charge on the sulfur here is zero. So I already did these calculations for you. If you sum up the formal charges we get minus one./nThe overall charge on this ion is minus one./nWe did it right. We did everything right. You should check your formal charges like this. Let's do the same thing for this structure. We calculate the formal charge on carbons minus two. The formal charge on sulfur is two./nThe formal charge on nitrogen is minus one. Let's sum these up. When we do that we get a minus one./nIt indeed matches the overall charge on the ion. Let's calculate the formal charges for each atom here./nSulfur is zero. Nitrogen is one. Carbon is minus two. Overall charge minus one. We did that right. Now, which one of these structures is the lowest energy structure? Ready? I'm going to ask you to raise your hand or to scream./nIs it structure number one? Yes./nIs it structure number two? No. Is it structure number three? No. You're right. It's structure number one. It has the lowest set of formal charges. Minus one, zero, zero. Only one atom here has a formal charge other than zero./nThese two structures have formal charges that are minus two or two./nI don't think that there is a structure on earth, well, maybe there is, that has a formal charge of minus two or two that will make it the lowest energy structure. If you've got a structure with a minus two or two, that's very, very likely not the lowest energy structure./nThis is a powerful tool to know if you have drawn your skeleton correctly, so remember this./nIt's really important. On an exam, we're going to tell you to draw the Lewis structure with the lowest energy. You're going to have to try several of them to see which one has the lowest set of absolute values for the formal charge./nQuestion?/nNumber of valence on the carbon is four minus the number of lone pairs on the carbon is four./nThat's two. Four, is that your question? Yeah. Other questions? OK. Here is another use of the formal charge./nThis is also determining the skeletal structure here, but you will see a problem in just a moment. Here is another special case. We have CH3NHO-./nHow do you draw, in this case here, that skeletal structure? Could I have some lights on the board here?/nCH3 here, this is what we call a methyl group./nIt is always terminal since the hydrogens are always terminal./nSo the structure looks like this. And the carbon then is bonded to the next element over. If you see a structure like this where you've got lots of different elements, the first pass just take these elements in a row./nThe CH3, this methyl group is bonded to the nitrogen./nThe next one is hydrogen, but we know we're not going to put the hydrogen between the nitrogen and the oxygen because the hydrogen is always terminal./nSo the hydrogen is either going to go here or the hydrogen is going to go here. And that's the question that we're going to ask here on this slide, is this the correct structure or is this the correct structure?/nI drew the skeletal structures, I drew the Lewis diagrams./nWhich one is right? Well, if I go and calculate the formal charges here, I would find that the formal charge on oxygen is minus one and that the formal charge on all other atoms is zero. In this structure, if I go calculate the formal charge on the nitrogen in this structure is minus one./nAnd then the formal charges on all the other atoms are zero./nWe've got a problem here. We've got two different structures, and they have absolutely the same set of formal charges in terms of the absolutely magnitudes. We have only one atom that has a minus one formal charge, only one atom that has one more electron that it usually does in its unbonded state, in its atomic state./nSo which one of these is correct? Well, it turns out that this structure is correct./nIt is correct because of this. It is correct because we put the formal charge on the most electronegative atom which is oxygen. Oxygen is more electronegative than nitrogen./nThat is the other key here, is that if you've got two structures, same sets of formal charges and you have a choice of putting the minus formal charge on an atom, you put it on the most electronegative atom./nThat is going to be the lowest energy structure. In general here we talked about the electronegativities. The electronegativity of fluorine is greater than oxygen, is greater than nitrogen, is greater than carbon./nThat is something that you will need to know./nSo we've seen how this formal charge now is useful in figuring out whether or not we had the right skeletal structure to begin with. Now, here is another --/n-- utility of formal charge. Let's look at this structure NO3-./nWell, I know ahead of time that we're going to put this nitrogen in the center. If you just had NO3- and you didn't know anything. You would have to play around with some structures and look at formal charges, but I have already done that playing around so I know to put nitrogen in the center./nBut let's do the Lewis diagram for this./nWe count up the valence electrons. We've got six for every one of the oxygens and five for the nitrogen. And now, this one is important, we added an extra electron when we counted up the valence electrons./nThat extra valence electron comes because this is an 03-. We have an extra electron on NO3 so we have to count it in our valence electrons. If this was NO3+, we would subtract one right here./nBut this is NO3- so we've got to add one./nThat's 24 valence electrons. Let me draw them all out. Number three. Let's calculate the number of electrons that we would have to have if each atom had a noble gas configuration. That would be 32 because we've got four atoms./nLet's calculate the number of bonding electrons./nWell, that's 32 minus 24 or eight. So, of those 24 valence electrons, eight of them are bonding electrons. Let's assign them. Well, here is two, here is two, there is two./nThat's great. We used up six. Do we have any remaining? We sure do. We've got two, so we're going to have to make one of these a double bond. So we've got one double bond there between nitrogen and oxygen./nNow, we've got a lot of remaining valence electrons./nWe've got 16 that are remaining. We're going to distribute them around the oxygens in order to make an octet around each atom. There we go. We did that. And so now we've used up all of our valence electrons./nLet's calculate the formal charges. The formal charge on the singly bonded oxygen is minus one./nI put a minus one there. I'm going to put a minus one there. The formal charge on the doubly bonded oxygen right in here, that's zero./nI'm going to put a zero there. The formal charge on the nitrogen, well, that's one. I'm going to put a one there. If I sum up the formal charges, I get a minus one. That does agree with the overall charge on this molecule, NO3-./nSo we did everything right./nBut now you see something a little strange here, right? And that is, for whatever reason, I picked this nitrogen and oxygen to make a double bond. But I could have also made the double bond between this nitrogen and oxygen./nAnd if I had calculated the formal charges, I would have identically the same set of formal charges. Or, I could have picked this oxygen and nitrogen to put in the double bond./nIf I calculated the formal charges, I'd have the same set of formal charges./nSo we've got three different structures all which have the same set of formal charges. What are we going to do here? Which one of these is correct? Well, when you've got this situation here, this is a hint that you've got what we call a resonance structure./nSo if you have the same skeletal arrangement and you've just go three different structures and the electrons are in different places and the same set of formal charges exist, this is a hint that you have something called a resonance structure./nWhat is a resonance structure? Well, here is the bottom line./nIn NO3-, if you look at the structure of that ion, you would not find that one of the nitrogen-oxygen bonds was a double bond and that the other two were single bonds./nA double bond is stronger than a single bond. A double bond is shorter than a single bond. So if you do the appropriate experiment you can tell whether or not you have a shorter or a longer bond./nYou can tell whether you have a weaker or a stronger bond./nThe bottom line is that when you look at this molecule experimentally, you do not see one bond that is shorter and stronger than the other two nitrogen-oxygen bonds. What you find is that all three bonds are equivalent./nAll three bonds have the same strength and the same length./nYou find that the length of the nitrogen-oxygen bonds are a little bit shorter than those of a single bond and you find that the nitrogen-oxygen bonds are uniformly, all three of them, a little bit stronger than a nitrogen-oxygen single bond./nBut not quite a double bond./nWhat you find is that all of these three bonds is like a bond and a third. So what is happening here? What is happening here is that this extra pair of electrons, instead of being localized on just one of the nitrogen-oxygen bonds, those extra pair of electrons are actually delocalized over all three bonds./nAnd we call that a resonance hybrid./nWe denote that resonance hybrid in this way, with three different structures with double-headed arrows. This does not mean, this is important, that the structure is flickering between here, here and here./nThat's not what is happening./nRather, these two extra electrons are delocalized over all three bonds such that the bond is like a bond and a third rather than one bond or two bonds. This is called a resonance structure, a resonance hybrid./nAnd you will be clued in that you've got this kind of resonance hybrid if you're able to write three structures with the same set of formal charges./nYou will know to do this. OK. Have a nice cool weekend./nSee you Wednesday.
Channels: Chemistry (General)
Uploaded by: mitlectures ( Send Message ) on 16-04-2009.
Duration: 45m 49s