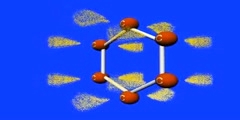
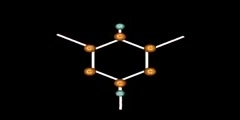
Resonance Structure of Benzene
This video shows how benzene is structured by resonance,orange is Carbon, the purple is the p-orbital, and the yellow dots are electrons. This animation is gives a better idea of how resonance occurs in a benzene/1,3,5 cyclohexatriene/2,4,6 cyclohexatriene molecule. Sigma bonds / s-orbitals localize around each atom respectively; whereas, the Pi-bonds are formed when electrons in the p-orbitals merge. For more of a science lesson, go to http://kchemistry.com/resonance.htm ...